Breast Cancer Spreading: A Team Effort?
Submitted by Seneca Cox on
Approximately every 13 minutes, in the United States alone, one woman will die of breast cancer. Metastasis, the main cause of patient death, is the process by which breast cancer cells spread throughout the body by means of blood and lymphatic vessels. Breast cancer cells metastasize primarily via the lymphatic vasculature, which is lined by endothelial cells. Previous research suggests that lymphatic endothelial cells (LECs) help tumors grow more quickly, but there is little information on whether, or how, the metabolism of breast cancer cells is impacted by the LECs. Unlike normal cell metabolism, which relies on oxidative phosphorylation, breast cancer cell metabolism relies on glycolysis and therefore consumes more glucose. In addition, altered signaling pathways in cancer cells allow for changes in cell metabolism that increase proliferation by supporting cell growth and survival. Interestingly, one study found that in the presence of LECs, breast cancer cells tend to switch to relying on oxidative phosphorylation and fatty acid oxidation instead of glycolysis. Other recent investigations have found that cancer cells rely on both glycolysis and oxidative phosphorylation for energy.
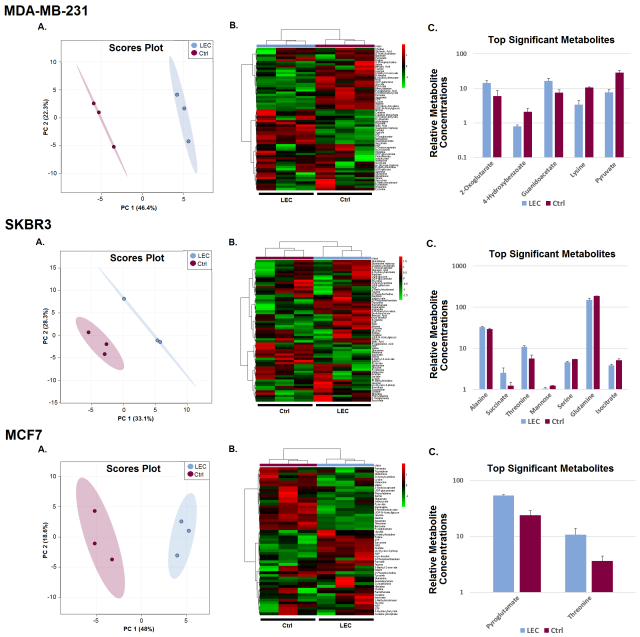
Given that few studies have been conducted, to develop a clearer understanding of LEC and breast cancer cell interaction we designed an experiment to determine if LECs affect the metabolism of breast cancer cells and how. Three breast cancer cell lines (MDA-MB-231, SKBR3, and MCF7) were co-cultured with LECs for four days. Breast cancer cells were co-cultured with themselves as a control. Using 1H NMR (nuclear magnetic resonance) metabolomics analysis, we identified and determined the concentration of the metabolites inside the breast cancer cells. Then, we used the MetaboAnalyst web server for various statistical tests on the data. By performing principal component analysis (PCA, Figure A) and hierarchical clustering (Figure B), we found that the concentrations of metabolites were notably different between the two cocultures. Thus, the LECs affected the metabolism of breast cancer cells. Results from a volcano test showed that the concentrations of many metabolites were significantly altered in the LEC co-culture relative to the control. These are shown in the bar graph (Figure C). The top significant metabolites for the MDA-MB-231 cells were 2-oxoglutarate, 4-hydroxybenzoate, guanidoacetate, lysine, and pyruvate. For the SKBR3s they were alanine, succinate, threonine, mannose, serine, glutamine, isocitrate. Pyroglutamate and threonine were the only two significant metabolites for the MCF7s. The variance in significant metabolites, suggests that each breast cancer cell line was affected differently.
Metabolite set enrichment analysis showed many of the core metabolic pathways, such as the TCA cycle and glycine, serine, and threonine metabolism, were enriched. This pathway analysis allowed us to better understand how the metabolism of the breast cancer cells were changed by highlighting which pathways were significantly altered in LEC co-culture relative to the control. The MDA-MB231s had the most significantly affected pathways. Thus, we suspect this breast cancer cell line is more susceptible to changes in metabolism due to co-culture with LECs. Due to changes in concentrations of pyruvate, 2-oxoglutarate, fumarate, and isocitrate, we suspect there was flux through the tricarboxylic acid (TCA) cycle for the MDA-MB-231s and SKBR3s co-cultured with LECs. No significant changes in glycolysis were observed in any of the co-cultures.
Since our results showed no change in glycolysis utilization, but enrichment of the TCA cycle in the MDA-MB-231 and SKBR3 breast cancer cells this may suggest that in the presence of LECs, MDA-MB-231 and SKBR3 breast cancer cell metabolisms rely more on the TCA cycle in addition to glycolysis. If LECs are indeed promoting oxidative phosphorylation, the combined sources of metabolic energy could possibly be facilitating the increase in metastasis and tumor growth observed in the previous studies. Confirming if and how LECs expedite cancer metastasis could lead to development of prevention methods or, possibly reversal of metastasis. Upon further analysis and validation, these results may allow for breakthroughs such as possible metabolic targets for cancer diagnostics or therapy development.