Making the Sun do our Dirty Work
Enviado por Austin John Dosh el
How can we use the sun’s energy to clean our water of hazardous materials? This summer, I have had the opportunity to study that question working in the labs at the Universidad de Puerto Rico Mayagüez (UPRM) doing research in the Civil Engineering Department, specifically in the Environmental area, under the mentorship of graduate student Alba Lacen and the advisement of Dr. Pedro Tarafa. There are a variety of dangerous chemicals that get into our water from many sources and removing pollutants becomes a problem for wastewater treatment plants to handle. Atrazine, an organic compound, is one such substance. Atrazine is a potent endocrine disruptor in humans and animals alike. Used extensively around the world as an herbicide, rainwater and irrigation eventually washes the highly effective weed killer off farmland and turf (such as golf courses) and into waterways. From waterways, it travels to treatment facilities, however, current methods for the removal of Atrazine from drinking water are both very expensive and largely ineffective.
One potential solution to remove Atrazine from drinking water may be to utilize Titanium Dioxide (TiO2). TiO2 is a semiconductor that can be used as a photocatalyst for the degradation of Atrazine. This means that, with the addition of energy, in the form of light, TiO2 breaks down Atazine, an organic molecule, into smaller and much more easily treated compounds such as water and Carbon Dioxide. An obstacle is that TiO2 is only effectivephotocatalytically when activated in the ultra violet light spectrum (less than 400nm). Of course, the sun emits energy at that wavelength also, but the photocatalyst could be much more effective if it was able to use more of the spectrum. Additionally, water absorbs UV light, so using it to treat contaminated water would be less efficient.
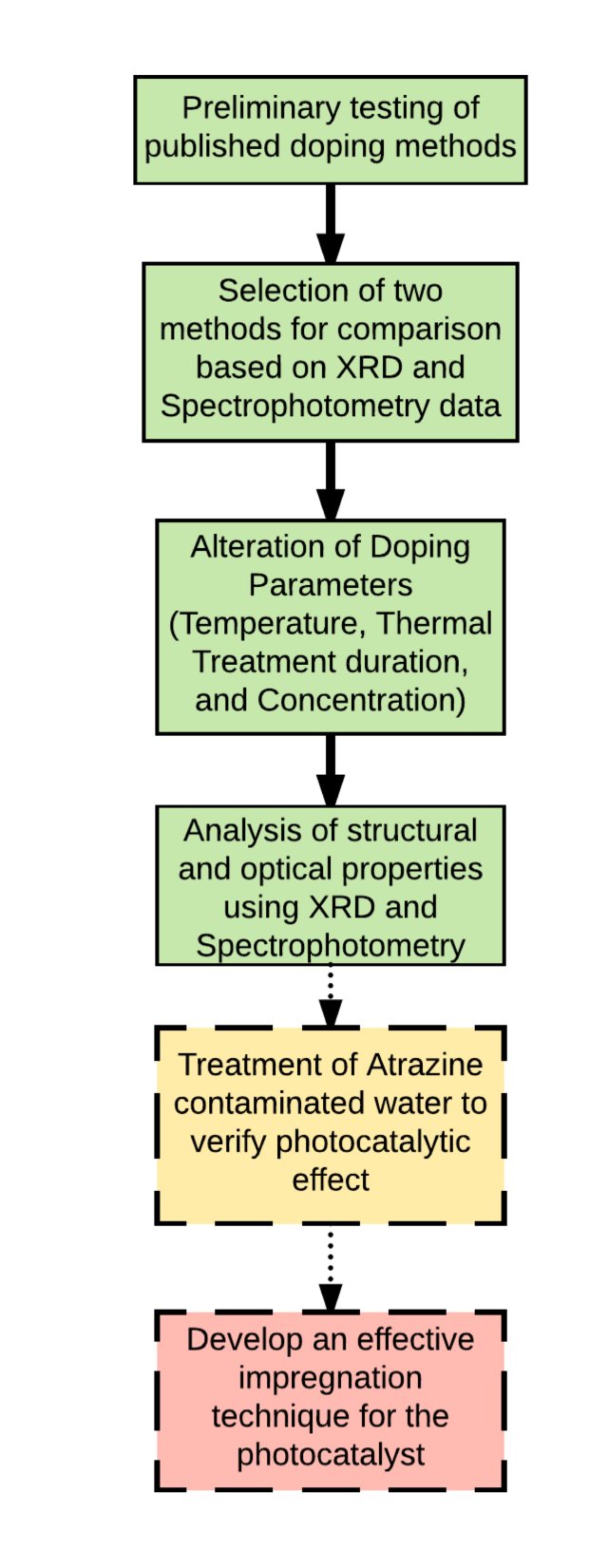
There is a way to raise the photocatalytic activation wavelength of TiO2 using Iron ions. Doping the TiO2 with Fe(III) can bring the activation wavelength into the visible spectrum. Doping is essentially when one type of particle situates itself comfortably in the structure of another crystalline substance. This is what my summer research has been primarily focused on. We have been studying various methods of doping TiO2 with Fe(III). My partner, Carlos Huang, has been focusing on preparing samples using pure titanium dioxide, while I have been working with a titanium dioxide precursor, titanium isoproproxide. We hypothesized that because, with the precursor, the crystalline lattice is not yet formed, it may behave differently when doped with Fe(III).
I worked with two different chemical reduction methods: starting with titanium isoproproxide and reducing to TiO2. With each doping method, 3 parameters were varied. The concentration of iron to titanium dioxide was varied between 2%, 4%, and 6%. The times spend in heat treatment (a step in the doping process) were varied between 2 hours, 4 hours, and 6 hours. Lastly, the temperature of the oven was varied between 450C, 600C, and 750C. Not counting the controls, there were 27 unique combinations done for both procedures. So 54 samples were prepared in total. Controls were prepared as well.
Two tests were used to analyze the samples. Photospectroscopy is a test that measures the absorbance of a solution based on the wavelength of light that is shone through it. By using this test, we determined the peak absorbance of the doped particles. This absorbance peak is expected to correlate to the peak wavelength of photocatalytic activation. (That expectation must be experimentally confirmed by degrading Atrazine with the doped particles.) Another test done on the doped particles was X-ray powder diffraction (XRD). XRD gives information about the physical properties of the particles. XRD tells us what phase the product is in.
Of the many samples tested, 450C heat treatment at lower concentrations seem to produce the best doped product, with absorbance peaks in the visible spectrum. Additionally, XRD results indicate that at temperatures of 750C, the structure of the TiO2 is completely converted to the Rutile phase. The Anatase phase is effective as a photocatalyst, but the Rutile phase is a structural change that results in an ineffective photocatalyst. Most of the XRD results are still pending, but based on preliminary results, 750C is not a viable treatment temperature.
Moving forward, once all the pending XRD data is in, the samples will be experimentally tested by degrading Atrazine with them. Once their effectiveness is determined the next step will be to determine a way to prevent the TiO2 from entering solution with the water by impregnating it on a surface. Then, beyond that, ways of making this process large scale will need to be looked at.
Unfortunately, as the research continues I will be returning to my state of Michigan and will not be a part of the next phase of research. This summer has been an incredible opportunity to get firsthand research experience in an interesting field with a very real problem to be solved. I am very grateful to the REU directors and professors for all they did to make this summer of research possible even in the face of difficulties. I am also thankful for all of the support and help provided by my lab partner Carlos, mentor Alba, and advisor Dr. Tarafa. I’m grateful to the NSF and to the many people who contributed to making this summer an incredible learning experience.