Bacteria infection is a real threat of orthopedic implants...
Submitted by Khoa Dang Do on
S. aureus is common gram-positive bacteria which may cause severe infections in clinical practices.In nature, bacteria normally exist in two states: planktonic or biofilms. Unlike planktonic cell, which are free floating microbes in suspension, cells within a biofilm adhere to the surface of soft tissues and implanted medical devices such as, hips and knees.Following bacterial initial attachment, the bacteria will secrete an extracellular polysaccharide matrix to protect themselves as they gather the accumulated nutrients from the surrounding aqueous environment required for biofilm survival.As a result, the bacteria within the biofilms are approximately 10 to 1000 times harder to be killed by antibiotics. Thus, implant failures often occur because of the increased tolerance of bacteria biofilms against antimicrobials, whichmight lead to re-implantation and high health expenditures. For this summer research, I used beta peptides, which are synthetic mimics of host-defense peptides in our immune system as an antimicrobial agent to prevent the S. aureus biofilm formation on the surfaces of orthopedic using Titanium (Ti) substrates as a model.
First, I coated the Titanium substrates with polyelectrolyte layers. The method was easy to do since it was dipping layer-by-layer in chitosan (CH) and hyaluronic acid (HA). After I got 19.5 bilayers, the Ti substrates was crosslinked in 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) and N-Hydroxysulfosuccinimide (NHS) solution overnight. By doing so, the films would be more stiffer and no rearrangement between the molecules would occur.
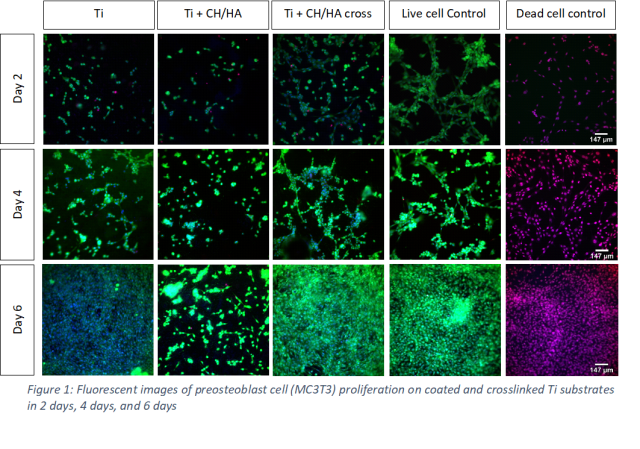
Follow that, I performed the attachment and proliferation experiment of preosteoblast (MC3T3) cells on our coated Ti substrates. The cells were seeded on top of each substrate in 2 days, 4 days and 6 days. Then, the Ti substrate would be taken to the dark room to take fluorescent images. Propidium iodide (PI), Hoechst, and Calcein were used as staining solution for the cells. As you can see in the picture, on the crosslinked substrates, more cell attachments was observed which suggested that crosslinking of CH/HA PEMs is essential for good MC3T3 attachment and proliferation.
Next, we were interested in how β peptide would react to MRSA bacteria cells and mammalian cells. So, we did the minimum inhibitory concentration (MIC) and 10 percent inhibitory concentration for MRSA and MC3T3 respectively. Our results suggest that peptide 1 successfully prevented S. aureus biofilm formation at concentrations between 4-8 μg/mL (Figure 4). However, when evaluating its biocompatibility using MC3T3 cells, we observed that peptide 1 causes 10% of mammalian cells death at a concentration of 16 μg/mL (Figure 5). As a consequence, these results shows that peptide 1 has a strong activity towards bacteria cells compared to preosteoblast cells.
Follow up, the MC3T3 attachment and proliferation on β peptide loaded and CH/HA-crosslinked coated Ti substrates was evaluated at 1, 2, 4 and 6 days. It was observed that the amount of β peptide was released over time prevents biofilm formation as the cell viability of bacteria goes down (Figure 7). In fact, at each time point, there were almost no cell viability per surface area on the peptide 1 (1mg/mL) loaded substrates. As a result, peptide 1 is determined to prohibit preosteoblast attachment and proliferation at 1 mg/mL loading concentration.
Then, the MC3T3 attachment and proliferation on β peptide loaded and CH/HA-crosslinked coated Ti substrates was evaluated after 24 hours in order to optimize the loading concentration of β peptide (Figure 8). We used β peptide at 1mg/ml, 0.5mg/ml and 0.25mg/ml as dipping solutions to load to the Ti substrates. As a result, attachment of mammalian cells was observed when we used the loading solution of 0.25 mg/ml of β peptide. On the other hand, on the Ti substrates that released peptide 1 at 1mg/ml and 0.5mg/ml, preosteoblast cell attachment and proliferation was prevented from Ti coated substrates.
For this experiment, biofilm was able to form at 0.25 mg/ml since the amount of peptide released at 0.25 mg/ml was not enough to prevent biofilm formation. In contrast, on the Ti substrates that released peptide 1 at 1mg/ml and 0.5mg/ml, biofilm formation was prevented from Ti coated substrates (Figure 9). Based on this result, further optimizing experiments will be performed to find the concentration range of peptide 1 in order to inhibit S. aureus biofilm formation without destroying mammalian microenvironment.
In conclusion. by using fluorescent imaging adhesion and proliferation of CH/HA crosslinked Ti substrates was studied, demonstrating that this biomaterial system doesn’t compromise Ti ability to allow MC3T3 attachment and further proliferation for 6 days period. Based on the results of β peptides activity against MRSA cells, peptide 1 has been showed to have an antimicrobial activity towards the S. aureus bacteria. Furthermore, the peptide 1 has a strong activity towards the bacteria compared to the mammalian cells based on the XTT and IC10 experiment results.
To be honest, I had not thought about how bad the situation of the patients who are struggling with implant infections before I started this project. However, 10 weeks working with mammalian cells and MRSA bacteria is kind of giving me the idea of how hard the treatments can be. I have failed so many attempts to get preosteoblasts cells attached to the Ti substrates as well as prevent biofilm from forming on those PEMs coatings. Nevertheless, I guess failing is the best way to learn new things. I want to say thanks to all the professors in both schools for making this internship experience happened. We all had great times in labs as well as hanging out with each other this summer.